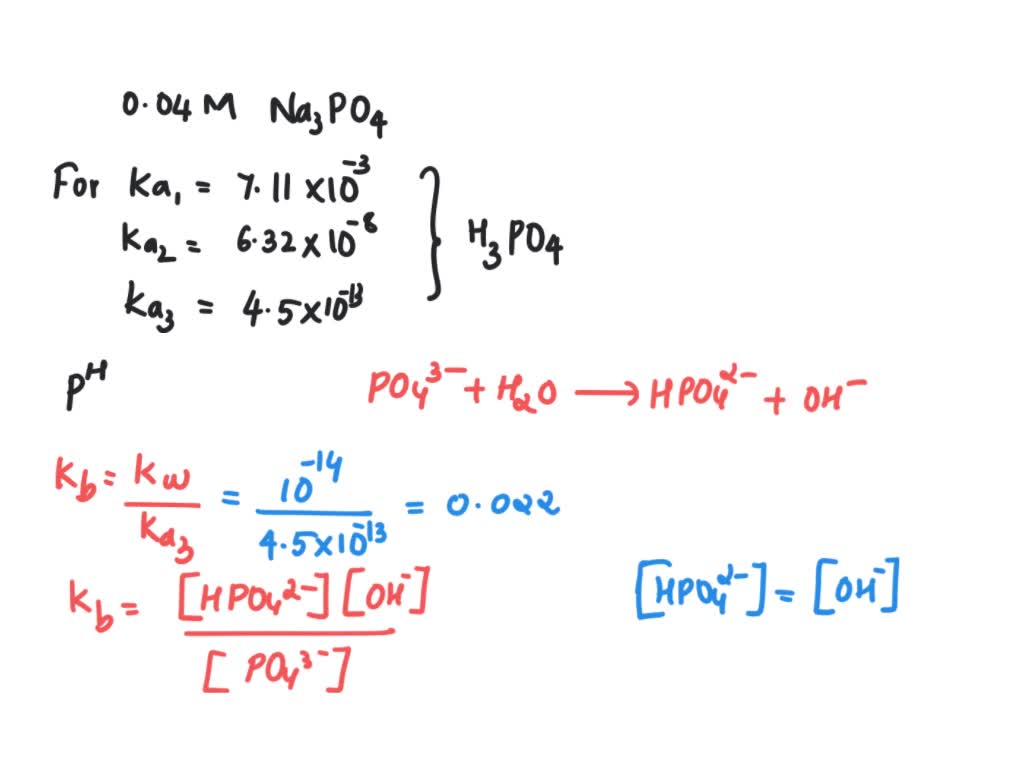
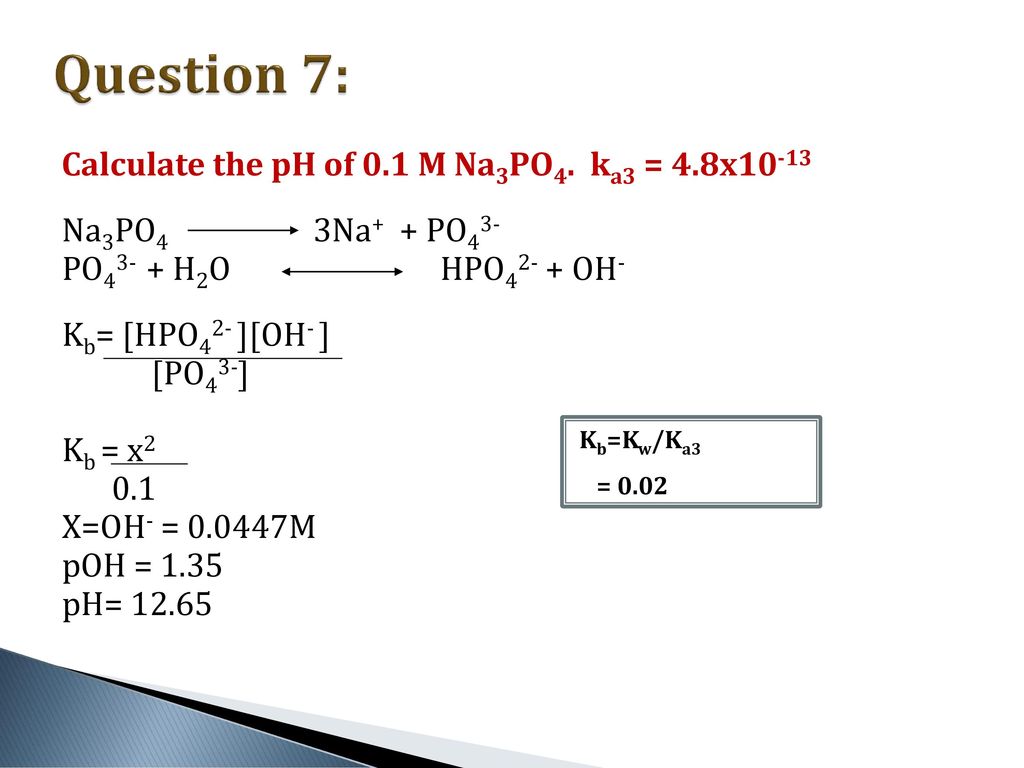
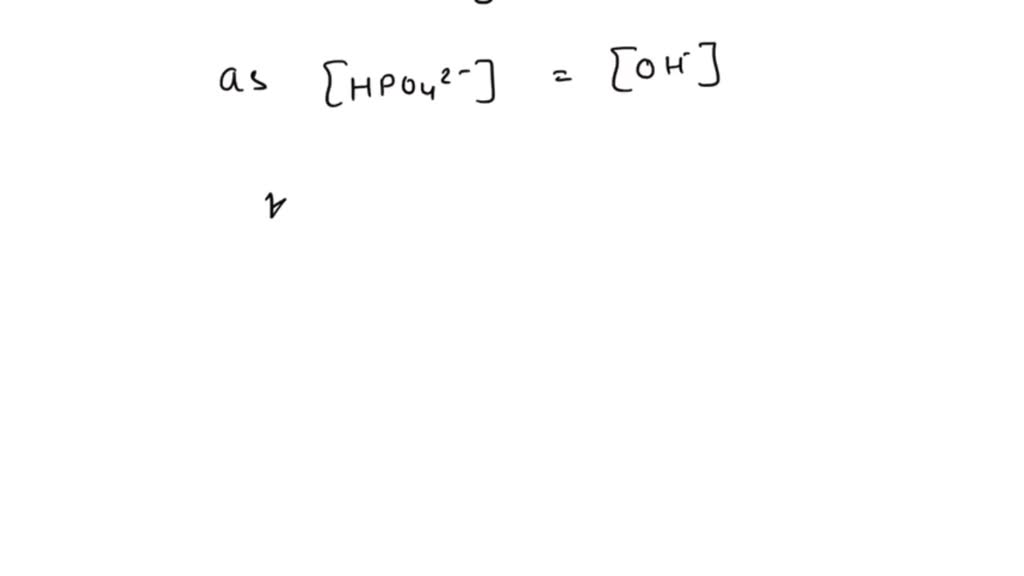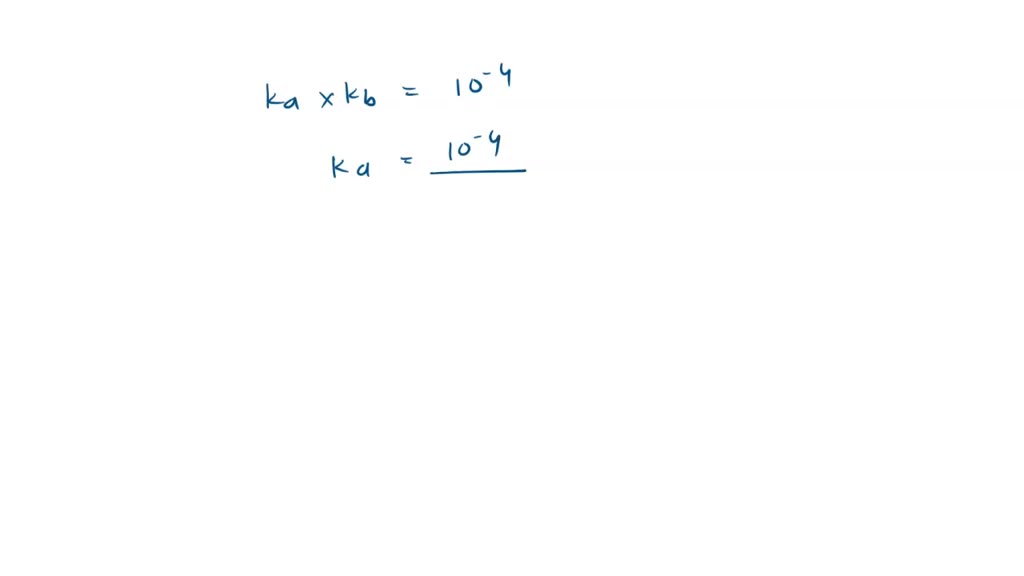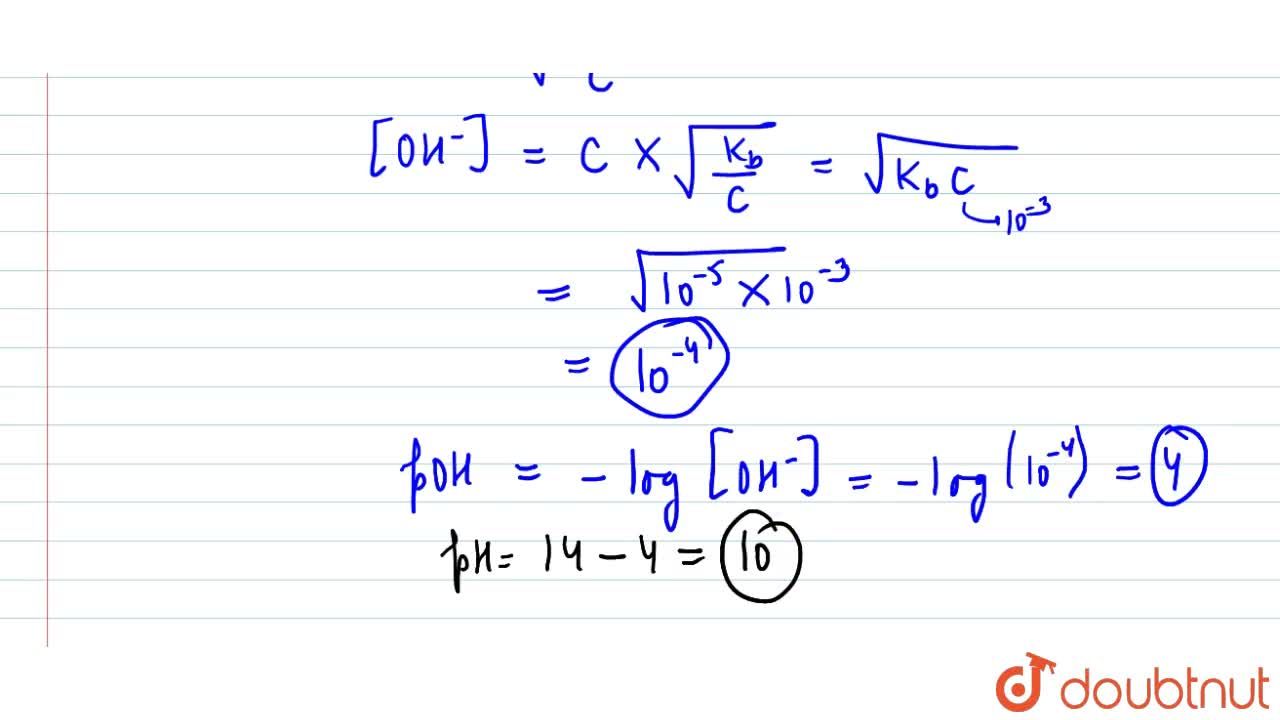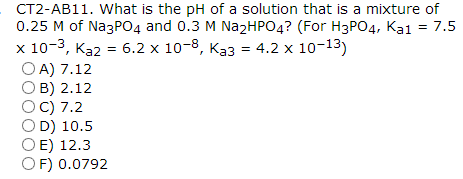Calculate the pH of 0.5 M Na3PO4 in aqueous solution ? - Sarthaks eConnect | Largest Online Education Community

If 2.5 moles each of H3PO4,NaH2PO4,Na2HPO4 and Na3PO4 are mixed together to form an aqueous solution, then the resulting pH is:Given values of Ka are: Ka1 = 10^-3 Ka2 = 10^-7 Ka3 = 10^-13
What is the pH of 1.0 M Na3PO4 in aqueous solution ? - Sarthaks eConnect | Largest Online Education Community

When 100 mL of 0.1 M KNO3 , 400 mL of 0.2 M HCl and 500 mL of 0.3 M H2SO4 are mixed, then in the resulting solution :