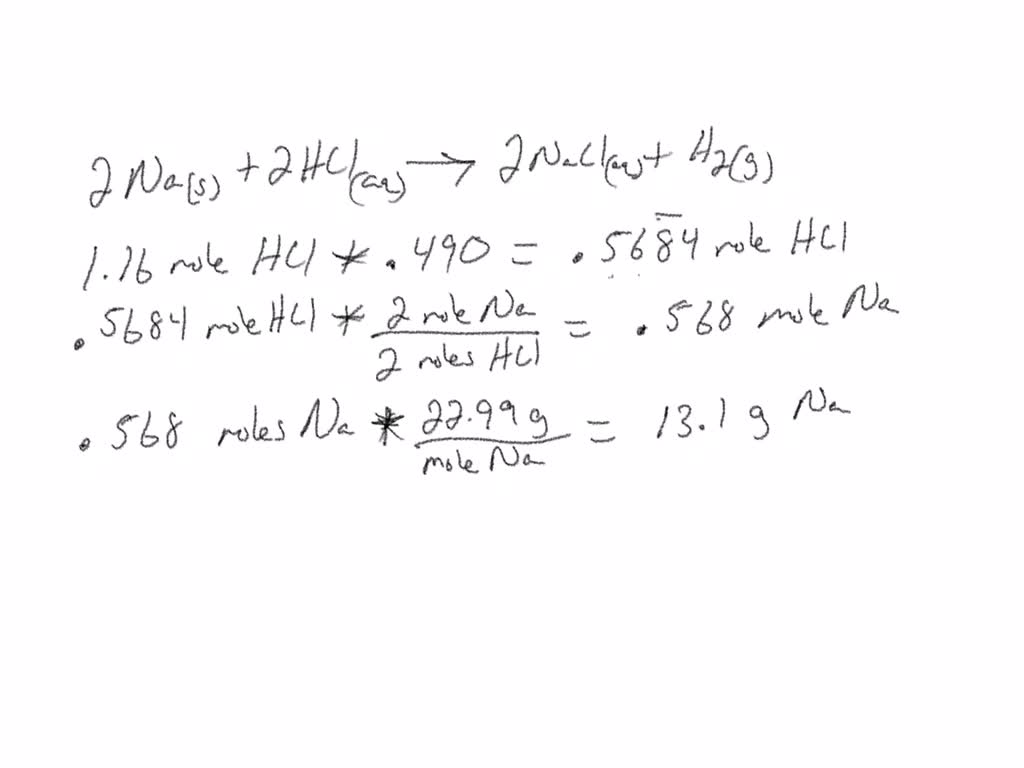
SOLVED: 2 Na(s) + 2 HCl(aq) → 2 NaCl(aq) + H2(g) If the aqueous hydrochloric acid solution contains 1.16 mol HCl, what is the amount of sodium that needs to be added

Write a chemical equation for the conversion of the following carboxylic acid salt to its parent carboxylic acid. Let hydrochloric acid (HCl) be the source of the needed hydronium ions. Sodium lactate.

SOLVED: How many mL of 0.250 M HCl would react exactly with 30.0 mL of the 0.150 M solution of Na(OH)2 solution? The chemical reaction involved is: HCl(aq) + NaOH(aq) → NaCl (

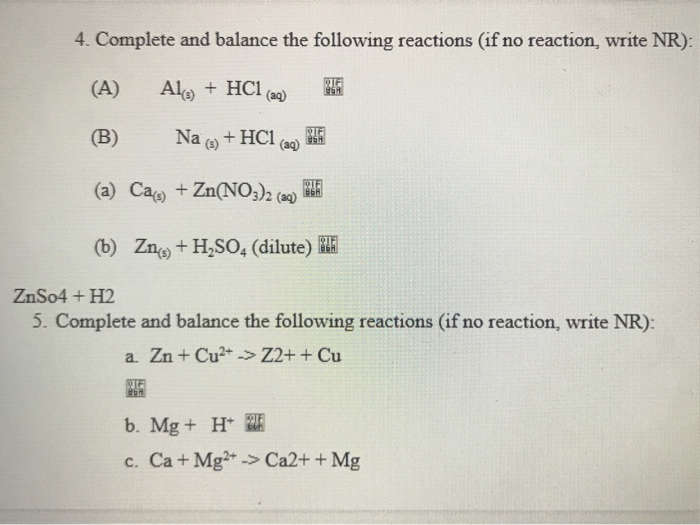
Ca , Na , Al , Cu , Pt , ile HCl ve NaOH tepkimesi ne oluır sırasıyla tepkimesi ne olur hepsi sırasıyla - Eodev.com
110ml of N/2 HCl, 20 ml of M/5 H2SO4,20 ml of N/4 H3PO4 are mixed together.The volume of water that must be added to make a 0.18N solution is(assume there is no